Products registration
Impeto Medical complies with State and Federal regulations regarding the manufacture and sale of SUDOSCAN and EZSCAN.
European Medical device directive 93/42 (class IIa) and ISO 13485:2016 delivered by SGS Belgium, notified body 1639.
Updated: december 2021
Products registration
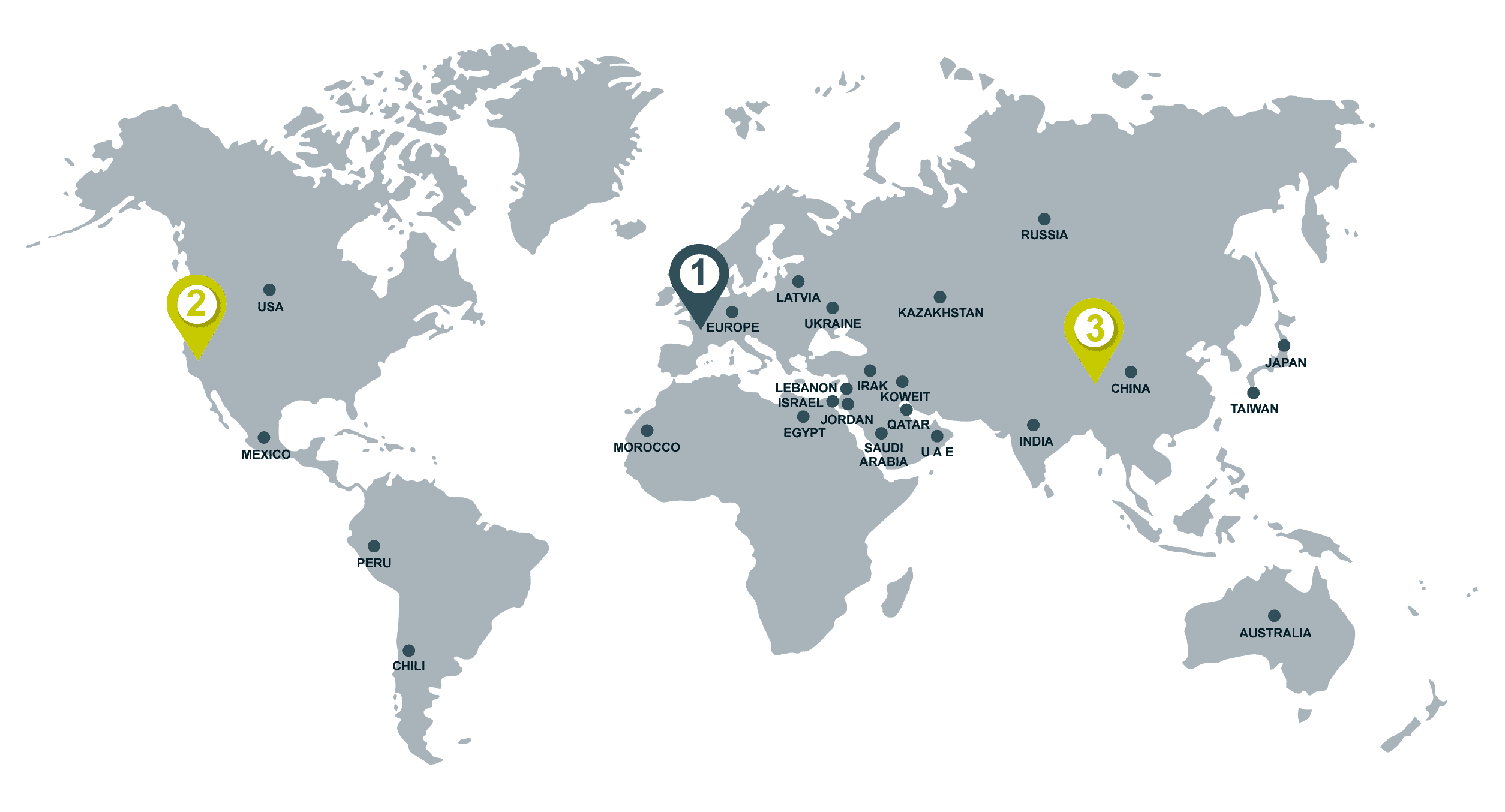
| Country | Products |
|
Europe (CE mark)
For : Belgium, Denmark, France, Germany, Italy, Luxembourg, Netherlands, Portugal, Romania, Switzerland, Spain, United Kingdom. |
SUDOSCAN EZSCAN |
| USA (FDA) | SUDOSCAN |
| Australia | EZSCAN |
| Chile | EZSCAN |
| China |
SUDOSCAN EZSCAN |
| Egypt |
SUDOSCAN EZSCAN |
| India |
SUDOSCAN EZSCAN |
| Irak | EZSCAN |
| Israel | SUDOSCAN |
| Japan | SUDOSCAN |
| Jordan |
SUDOSCAN EZSCAN |
| Kazakhstan |
SUDOSCAN EZSCAN |
| Kuwait |
SUDOSCAN EZSCAN |
| Lebanon |
SUDOSCAN EZSCAN |
| Latvia |
SUDOSCAN EZSCAN |
| Mexico | SUDOSCAN |
| Morocco |
SUDOSCAN EZSCAN |
| Peru | SUDOSCAN |
| Qatar |
SUDOSCAN EZSCAN |
| Russia |
SUDOSCAN EZSCAN |
| Taiwan |
SUDOSCAN EZSCAN |
| Saudi Arabia |
SUDOSCAN EZSCAN |
| Ukraine | SUDOSCAN |
| United Arab Emirates |
SUDOSCAN EZSCAN |